New York's highest court of appeals has held that no-fault insurers cannot deny no-fault benefits where they unilaterally determine that a provider has committed misconduct based upon alleged fraudulent conduct. The Court held that this authority belongs solely to state regulators, specifically New York's Board of Regents, which oversees professional licensing and discipline. This follows a similar recent ruling in Florida reported in this publication.
Spinal Steroid Injections: Assessing Risks vs. Benefits
A patient tells you her family medical doctor has recommended a steroid injection for sciatica. Her doctor told her she has disc herniation and degeneration, and that the steroid injection will help with the pain and inflammation. This patient is in her mid-50s and is underweight.
How do you respond? What does the current research demonstrate? Let's investigate the risks and benefits of steroid spinal injections, starting with the risks.
Steroid Injections: The Risks
A case-control study assessed the effects of lumbar epidural steroid injections on the risk of subsequent vertebral body fracture. This study was published in the Journal of Bone & Joint Surgery, a top-tier peer-reviewed journal for orthopedists.1 The research team consisted of five medical researchers and the principal investigator, an orthopedic spine surgeon.
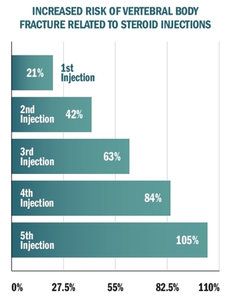
Let's take a look at the study's patient population. This research team randomly selected 3,000 patients who had been exposed to steroid injection and a matched cohort of 3,000 patients who had not been exposed. There were no differences between the injected and non-injected groups with respect to age, sex, race, hyperthyroidism or oral steroid use. The researchers assessed the incidence of vertebral body fractures in each group.
The graph titled, "Increased Risk of Vertebral Body Fracture Related to Steroid Injections," demonstrates the conclusions of this study. Each steroid injection increases the risk of fracture by 21 percent.
This is the first scientifically rigorous effort to quantify the fracture risk associated with spinal steroid injections. But it is not the first study to explore this issue. Another study examined postmenopausal women and demonstrated diminished bone density in the hip after a single lumbar steroid injection.2 Multiple research teams now caution against using these injections in women and underweight individuals.
Numerous other adverse events are associated with steroid injections. Although the vast majority are minor and transient in nature, serious complications, including nerve damage, paralysis, strokes and death, may also result.3
Infection is another possible risk that should not be ignored. Several years ago, an outbreak of fungal meningitis from tainted steroids killed 64 people and seriously sickened 751 others.
Steroid Injections Have Failed to Demonstrate Benefit
Scientists use double-blind, placebo-controlled, randomized clinical trials to examine the benefits of interventions because this research design can accurately measure effects. Combining the results of multiple trials in a meta-analysis adds a level of reliability to the conclusions of the individual studies.
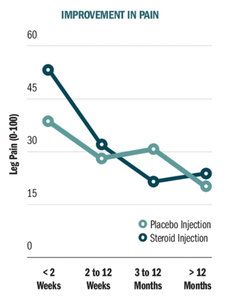
Oliveira and his research team conducted systematic review and meta-analysis to investigate the efficacy of epidural corticosteroid injections compared with placebo injections in reducing leg pain (primary outcome) and disability in patients with sciatica.4 They included in their review 25 placebo-controlled, randomized clinical trials that provided data from a total of 2,470 sciatica patients.
The conclusions of this review are demonstrated in the graph titled, "Improvement in Pain." The researchers found that while epidural steroid injections are more effective than placebo injections in reducing leg pain immediately after injection (e.g., the first two weeks), the effect fades rapidly. Epidural steroid injections were not better than placebo injections for leg pain at any point after two weeks. Additionally, epidural steroid injections were not effective in reducing back pain or improving disability at any point in time.
Multiple medical research teams performing independent systematic reviews agree with Oliveira, et al.5-6 They have concluded that there is no evidence to support the use of injections for the treatment of low back pain, spinal stenosis or sciatica. Although some patients will experience a few weeks of pain relief, the benefit does not last. Based on multiple randomized trials, steroid injections are not better long term than placebo injections with saline solution.
Clinical Takeaway
In the case of spinal steroid injections, the risk of harm is considerable, serious and predictable, and the benefits are extremely limited (14 days). Therefore, you should recommend that patients with sciatica, low back pain or spinal stenosis avoid this invasive treatment and instead receive chiropractic care.
Please note that the FDA has not approved injectable corticosteroids for use through spinal injection, but these products continue to be used off-label. Unfortunately, the FDA has not stated that corticosteroids are contraindicated, which would forbid their use for spinal conditions. However, based upon the data to date, the FDA should ban injectable steroids for spinal conditions.
Author's Note: The research presented in this article is also available in video format at https://chiroevidence.com/research-capsule-240.
References
- Mandel S, Schilling J, Peterson E, et al. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg (U.S.), 2013;95:961-4.
- Al-Shoha A, Rao DS, Schilling J, et al. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine, 2012;37:E1567-71.
- Abbasi A, Malhotra G, Malanga G, et al. Complications of interlaminar cervical epidural steroid injections: a review of the literature. Spine, 2007;32:2144-51.
- Oliveira CB, Maher CG, Ferreira ML, et al. Epidural corticosteroid injections for sciatica: an abridged Cochrane systematic review and meta-analysis. Spine, 2020;45:E1405-E1415.
- Chou R, Hashimoto R, Friedly J, et al. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta-analysis. Ann Intern Med, 2015;163:373-81.
- Pinto RZ, Maher CG, Ferreira ML, et al. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta-analysis. Ann Intern Med, 2012;157:865-77.



