It’s a new year and many chiropractors are evaluating what will enhance their respective practices, particularly as it relates to their bottom line. One of the most common questions I get is: “Do I need to be credentialed to bill insurance, and what are the best plans to join?” It’s a loaded question – but one every DC ponders. Whether you're already in-network or pondering whether to join, here's what you need to know.
Treating Neuropathies With Low-Intensity Laser Therapy
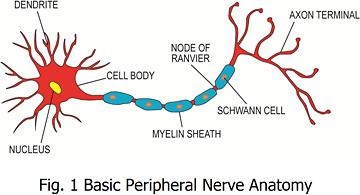
Peripheral neuropathy is defined as damage to the peripheral nervous system resulting in a syndrome of sensory loss, muscle weakness and atrophy along with vasomotor symptoms, alone or in any combination.1 The basic peripheral nervous system components consist of a cell body located in either the anterior horn (motor) or the dorsal root ganglia (sensory) of the spinal cord and a long extension (axon) covered in a chain-like series of cells known as Schwann cells; these produce myelinated nerve fibers.2 (See Figure 1.)
The thin, microscopic nerve fibers are surrounded by connective tissue referred to as the endoneurium. Bundled nerve fibers (fascicles) are then surrounded by a strong connective tissue known as perineurium. Finally, loose connective tissue called epineurium surrounds groups of bundles or fascicles.
Thirty-one paired spinal and 10 cranial nerves form the basis of the more numerous peripheral nerve trunks and their terminal branches. Peripheral nerves are highways for the conduction of nerve impulses to and from the spinal cord. The larger the diameter of the nerve axon (i.e., width of the highway), the faster the impulses can be conveyed. For example, the sensations of light touch and vibration move quite rapidly along very large axons, while pain and temperature sensation move more slowly along smaller axons in their course toward the spinal cord. Motor nerves have large-diameter axons that rapidly convey impulses away from the spinal cord to the skeletal muscles.
Classification
According to the National Institute of Neurological Disorders and Stroke, there exist over 100 different types of identified peripheral neuropathies. The causes include toxic and metabolic factors, infectious diseases, mechanical compression, inflammation, ischemia and paraneoplastic lesions. In developed countries, diabetes and alcoholism are the most frequent causes of peripheral neuropathy.3 The common incidence of these conditions can be time consuming from a diagnostic perspective, therapeutically frustrating and economically burdensome.
Generally speaking, the various types of neuropathy can be classified into three basic patterns of distribution: single peripheral nerve (focal mononeuropathy), multiple single peripheral nerves (multifocal mononeuropathy) and peripheral nerves simultaneously (symmetrical polyneuropathy). More specialized sub-patterns of peripheral neuropathy can include both an affected nerve root (radiculopathy) and the nerve plexus (plexopathy).
When classifying peripheral neuropathies, the time, course and presenting fiber deficit (sensory, motor or mixed), along with the pattern of distribution, are important factors that must be considered. For example, peripheral neuropathies may present as an acute (less than three weeks) purely motor neuropathy (e.g., Guillain-Barre syndrome) or chronic (more than 8-12 weeks) symmetrical purely sensory polyneuropathy (e.g., diabetes mellitus). In addition, there are mixed sensory and motor neuropathies that may or may not follow a specific dermatomal pattern (carpal tunnel, disc herniation, etc.). In the above examples, both large- and small-fiber (diameter) axons are equally affected. In rare cases, small-fiber neuropathy presents exclusively, creating distal impairment of pain and temperature sensations only.
Clinical Manifestation and Pathophysiology
Clinical signs and symptoms of peripheral neuropathy depend primarily on the etiological factors involved. In this review, the focus is on compressive neuropathies. Compression of a normal, healthy nerve results in paraesthesias and muscle weakness, generally without pain. On the other hand, compression of an inflamed nerve can cause pain, in addition to the objective neurological findings.4 Compressive neuropathies frequently occur as the nerve passes through tight pathways or tunnels formed by the encasing tissue borders.
Examples of compression include the median nerve at the wrist, the ulnar nerve at the wrist or elbow, and more commonly, spinal nerve roots traversing the intervertebral foramen. Nerve roots are more susceptible to compression compared to peripheral nerves due to their less developed vascular network and lack of epineurium (outer connective tissue covering).5Compressive neuropathies involve nerve dysfunction secondary to localized interference of microvascular function and structural changes in the nerve or adjacent tissues.6 As nerve tissue is compressed, pressure gradients develop, forcing tissues into areas of lower pressure. Early symptoms are primarily due to transient changes to microcirculation associated with edema and often result in morphological changes, including segmental demyelination (chronic impairment of microcirculation).6 If nerve compression persists, distal axonal degeneration of nerve fibers occurs, manifesting as persistent paraesthesias (numbness/tingling), muscle weakness and atrophy.
Finally, nerve compression appears to be both rate and pressure dependent, with acute duration resulting in only brief vascular interruption, while chronic sustained pressure results in both microvascular edema and permanent structural damage.6
Focal Mononeuropathy (Carpal Tunnel)
Carpal tunnel is primarily a compressive neuropathy. Patients may initially present with intermittent numbness and tingling in the digits, occurring primarily at night. The pain and tingling may radiate proximally and numbness generally affects the digits according to the median nerve distribution.7 If compression persists or progresses, more severe symptoms may occur. These include paraesthesias and numbness, which can result in muscle weakness and atrophy.
Radiculopathy (Nerve-Root Compression)
Radiculopathy is generally caused by compression of the nerve root(s), as demonstrated in degenerative disc disease, disc herniation, spinal stenosis etc.; this can result in paraesthesias and pain along the dermatomal distribution of the affected nerve root. For example, a lumbar disc herniation affecting the L5 nerve root will cause pain over the lateral thigh and dorsum of the foot with potential loss of sensation, specifically in the interspace between the first two toes, and eventually a decrease in the power of dorsi-flexion of the foot.
Compression of a deep branch of the peroneal nerve (mononeuropathy) at the inferior aspect of the extensor retinaculum causes a somewhat similar loss of sensation as an L5 radiculopathy, but no loss in the power of dorsiflexion of the foot.
Lumbar spinal stenosis often causes the syndrome of activity related thigh and leg pain (uni- or bilateral) associated with low back pain. Bilateral radicular symptoms, such as numbness and weakness in the legs and feet, occur with activity (e.g., walking) and are often relieved by rest (sitting, leaning forward). The compressive symptoms of LSS cause neural ischemia and neurogenic claudication.
Low-Intensity Laser Treatment
Non-conservative treatment (surgical decompression) to remove the mechanical extraneural pressure has been shown to only be moderately successful in severe cases. For example, a recent systematic review found surgery for spinal stenosis to be associated with only short-term benefit compared to nonsurgical therapy, with benefits diminishing over long-term follow-up in some trials.8 It has been demonstrated conclusively that neurological symptoms can persist even after the mechanical pressure has been removed.9
Further, documented damage to nerves follows a dose-dependent response sufficient to cause axonal degeneration, which may not be reversed by simply relieving the mechanical pressure. As compressive neuropathies span a continuum from the early phase of impaired blood flow and inflammation to eventual axonal degeneration, a treatment method targeting both the inflammatory process and regeneration is desirable from a result-based perspective.
Take-Home Points
|
A double-blind randomized study found an almost 70 percent vs. 18 percent improvement in positive somatosensory-evoked responses and better quality (larger-diameter axons) of the nerve regeneration process with LILT after complete surgical transection and direct anastomosis of the sciatic nerve.11 Clinically, a study by Iijima, et al. (1991) using LILT reduced pain levels by 45 percent in 18 patients with severe post-herpetic neuralgia.12
A clinical double-blind, placebo-controlled, randomized study compared the effectiveness of LILT on patients who had been suffering (six months to several years) from incomplete peripheral nerve and brachial plexus injuries. This study revealed that LILT significantly improved motor function in addition to recruitment of voluntary muscle activity in the partially paralyzed limbs of brachial plexus injuries compared to the placebo group, which failed to show any improvement.
More specifically, a randomized controlled trial carried out at General Motors Company found that carpal tunnel patients treated with low-intensity laser had better functional recovery and a higher back-to-work percentage (72 percent active laser vs. 41 percent sham).13 Similarly, a more recent studyshowed that LILT improved both the sensory and distal motor latencies of the median nerve in carpal tunnel patients compared to controls.14
In short, by safely and effectively healing damaged nerve tissue, restoring function and alleviating pain, low-intensity laser has been found to be a key therapeutic component in the treatment of peripheral nerve injuries.
References
- Rubin M. Peripheral neuropathy: peripheral nervous system and motor unit disorders. Merck Manual, February 2008.
- Rempel D, Tittiranonda P, Burastero S, Hudes M, So Y. Effect of keyboard keyswitch design on hand pain. J Occup Environ Med,1999;41(2):111-9.
- Poncelet AN. An algorithm for the evaluation of peripheral neuropathy. Am Fam Physician, 1999 Feb 15;57(4):755-64.
- Garfin SR, Herkowitz HN, Mirkovic S. Spinal stenosis in instructional course lectures. J Bone Joint Surg, 1999;81:572-86.
- Takahashi K, Shima I, Porter RW. Nerve root pressure in lumbar disc herniation. Spine, 1999;24(19):2003.
- Rempel D, Dahlin L, Lundborg G. Pathophysiology of nerve compression syndromes: response of peripheral nerves to loading. J Bone Joint Surg Am, 1999 Nov;81(11):1600-1610.
- Hughes RA. Treating nerves: a call to arms. J Peripher Nerv Syst,2008 Jun; 13(2):105-11.
- Chou R, Baisden J, Carragee EJ, Resnick DK, Shaffer WO, Loeser JD. Surgery for low back pain: a review for the evidence for an American Pain Society Clinical Practice Guideline. Spine, 2009 May 1;34(10):1094-109.
- Lundborg G, Myers R, Powell H, Nerve compression injury and increased endoneural fluid pressure: "a miniature compartment syndrome." J Neurol Neurosurg Psychiatry, 1983 Dec;46(12):1119-24.
- Rochkind S. Photoengineering of neural tissue repair processes in peripheral nerves and the spinal cord: research development with clinical applications. Photomed and Laser Surg, 2006 Apr;24(2):151-7.
- Shamir MH, Rochkind S, Sandbank J, Alon M. Double-blind, randomized study evaluating regeneration of the rat transected sciatic nerve after suturing and post-operative low-power laser treatment. J Reconstr Microsurg, 2001 Feb;17(2):133-7.
- Iijima K, Shimoyama N, Shimoyama M, Mizuguchi T. Evaluation of analgesic effect of low-power He-Ne laser on post-herpetic neuralgia using VAS and McGill Pain Questionnaire. J Clin Laser Med Surg,1991 Apr;9(2):121-6.
- Anderson T, Good W, Smith C, Vangsness CT, Treatment of repetitive use carpal tunnel syndrome: a General Motors study. Proc SPIE, 1995;2395:658.
- Evcik D, Kavuncu V, Cakir T, Subasi V, Yaman M. Laser therapy treatment of carpal tunnel syndrome: a randomized control study.Photomed Laser Surg, 2007 Feb;25(1):34-9.


