Some doctors thrive in a personality-based clinic and have a loyal following no matter what services or equipment they offer, but for most chiropractic offices who are trying to grow and expand, new equipment purchases help us stay relevant and continue to service our client base in the best, most up-to-date manner possible. So, regarding equipment purchasing: should you lease, get a bank loan, or pay cash?
How Diet Can Feed Inflammation, Pain, and Chronic Disease
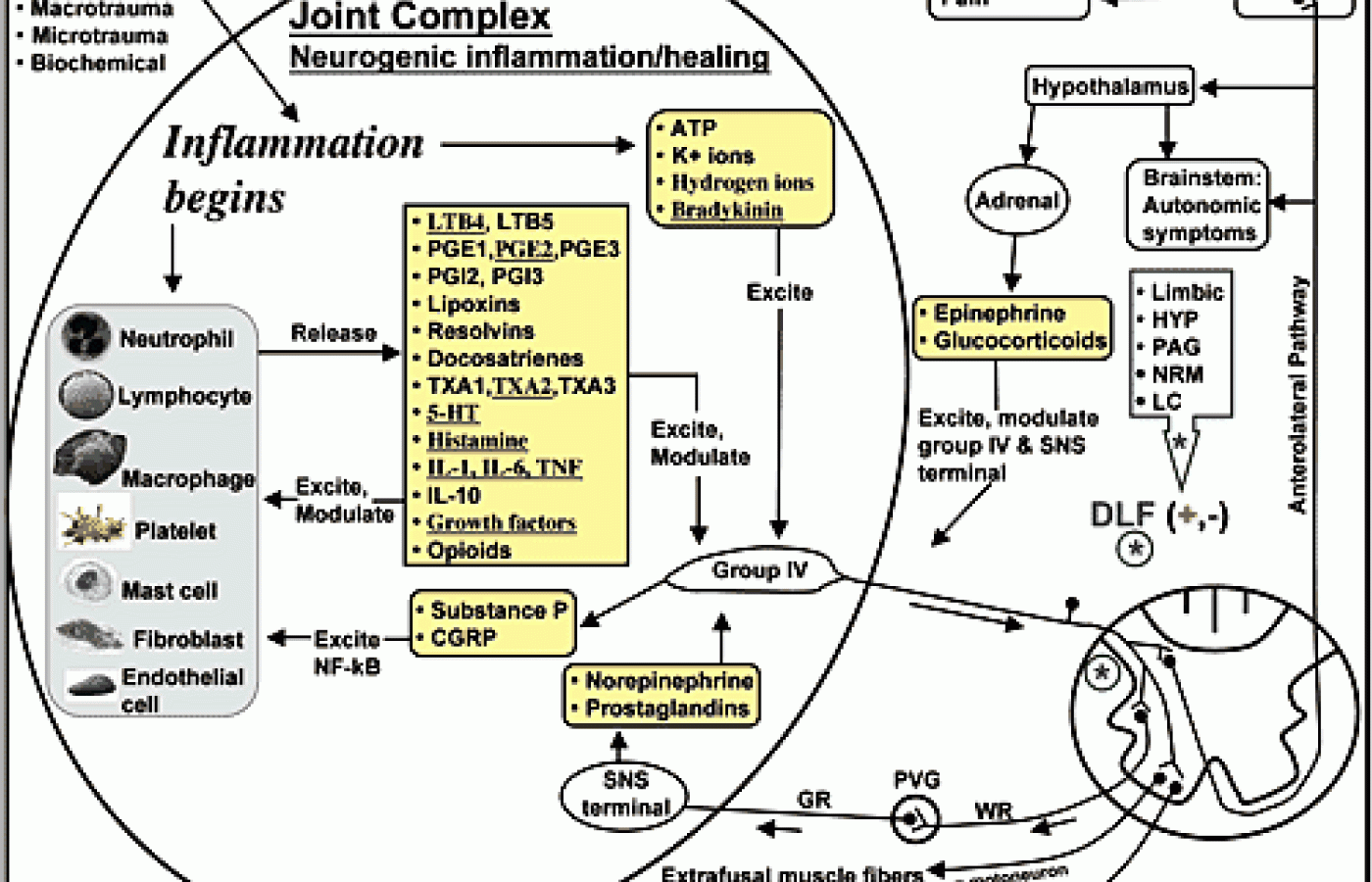
Neurogenic inflammation is thought to be involved in the pathogenesis of numerous conditions, such as osteoarthritis, migraine, dental disease, pancreatitis, virus-associated respiratory infection, nonproductive cough, allergic rhinitis, asthma, chronic bronchitis, sarcoidosis, inflammatory bowel disease, rheumatoid arthritis, and painful conditions in general.1-7 The figure at right represents a simplified version of the neurogenic inflammatory process. Immediately upon injury, damaged cells release various chemical mediators, and thereafter, intact cells in the area of injury produce many more mediators. Each of these mediators has receptors on group IV afferent fibers, which leads to the excitation or inhibition of the sensory nerve fibers typically associated with nociception and pain. One outcome of such stimulation can be chronic inflammation, pain, and the expression of the many diseases mentioned earlier.
Not generally appreciated is that group IV afferents also function to restore local tissue homeostasis and healing.8 Group IV afferents merely respond to the local chemical environment. I should emphasize that it is ahuge error in thinking to assume that nociceptors only function to create pain. Nociceptors are extremely diverse in nature. Indeed, world-famous pain researchers understand better than the rest that group IV are not pain fibers:
"A nociceptor is not born to be and to remain that kind of fellow forthe rest of his life. It is a dynamic entity, shifting its properties underthe influence of, and according to, the necessities of itsenvironment." 9
Notice in the figure that group IV afferents release substance P and CGRP (calcitonin gene-related peptide), which serve to stimulate local tissue cells. At this point in the neurogenic inflammatory process, chronic pain and disease would not be present. This is because substance P and CGRP are not themselves responsible for driving inflammation. Indeed, substance P, in particular, is often mischaracterized as an inflammatory chemical.
The pain, disease or healing outcome from substance P/CGRP release seems to be determined by the pro-inflammatory or anti-inflammatory nature of the chemicals released by the local cells. Notice the long list of mediators that local cells release; some promote inflammation and the diseases mentioned earlier, while others reduce inflammation and promote tissue healing - and nearly all of the mediators are regulated by diet. In particular, the eicosanoids or autocoids are derived exclusively from the fatty acids we consume. The leukotrienes (LTB), prostaglandins (PGE), prostacylins (PGI), lipoxins, resolvins, docosatrienes, and thromboxanes (TXA) are derived from either omega-6 (n-6) or omega-3 (n-3) fatty acids.
Humans are supposed to consume a 1:1 ratio of n-6s to n-3s; however, we currently average about 10:1 to 30:1.10 Anything above 4:1 is thought to be pro-inflammatory; this is because an increased level of dietary n-6 fatty acids leads to an increased production and release of pro-inflammatory autocoids from local cells (LTB4, PGE2, PGI2, TXA2).10 The omega-6 fatty acids and their autocoids also facilitate the release of some of the other pro-inflammatory chemicals listed in the figure, such as serotonin (5-HT), histamine, interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor (TNF), and growth factors.10
After the pro-inflammatory mediators are released, they again stimulate the local group IV afferents, which respond by releasing more substance P/CGRP - and thus, the cycle continues. Taking NSAIDs can only temporarily inhibit the conversion of pro-inflammatory fatty acids into their respective pro-inflammatory autocoids. After NSAID levels drop, we are left with a 30:1 ratio of n-6s to n-3s, which perpetuates the chronic inflammation cycle. We use the terms "inflammation" and "neurogenic inflammation;" however, "diet-driven inflammation" is a term that more accurately characterizes the problem.
Patients need to be told that their diet can either feed the healing process or feed chronic inflammation and the disease process. Group IV afferents can be referred to as pain fibers when conversing with patients who suffer from pain, and so these patients should be told that their diet functions to feed the pain fibers, leading to seemingly endless chronic pain.
Patients also need to know that correcting n-6 imbalances is not something a doctor can do for a patient. Only the patient can reduce his or her consumption of n-6s and increase n-3s, and this requires a disciplined approach to diet and supplementation. For example, consider that only fruits, vegetables, and potatoes have n-6:n-3 ratios below 4:1. Most fish, grass-fed animals and wild game have ratios approximately 4:1 or better. Fats/oils with acceptable ratios or low levels of n-6s include butter, olive oil, and coconut oil.11-12
Grains, grain flours, peanuts, and seeds have ratios of 20:1 or greater. Soy has a ratio of 7:1. Potato chips are oiled with corn, sunflower, cottonseed, or safflower oil, and these oils have n-6:n-3 ratios that range from 70:1 to over 100:1.11-12 These seed oils and the many packaged foods that are literally"bathing" in these oils feed the inflammatory process.
We and our patients should eat almost exclusively those foods with proper n-6:n-3 ratios. However, in today's fast-paced and inflamed society, it is likely that we are all going to come up short of our goal. For this reason, we all need to be supplementing with at least 1 gram of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from fish oil.
References
- Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation.Rheumatology 2005;44:7-16.
- Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology 2005;64(Suppl 2):S9-S15.
- Spierings EL. Pathogenesis of the migraine attack. Clin J Pain 2003;19:255-62.
- Lundy FT, Linden GJ. Neuropeptides and neurogenic mechanisms in oral and periodontal inflammation. Crit Rev Oral Biol Med 2004;15(2):82-98.
- Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology 2004;4:551-60.
- O'Connor TM, O'Connell J, O'Brien DI, et al. The role of substance P in inflammatory disease. J Cell Physiol 2004;201:167-80.
- Brack A, Rittner HL, Stein C. Neurogenic painful inflammation. Curr Opin Anaesthesiol 2004; 17:461-64.
- Byers MR, Bonica JJ. Peripheral Pain Mechanisms and Nociceptor Plasticity. In: Loeser JD (editor). Bonica's Management of Pain, 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001: p.26-72.
- Schmidt RF, Schaible HG, Mefslinger K, et al. Silent and active nociceptors: structure, functions, and clinical implications. Proc 7th World Cong Pain. Prog Pain Res Manag, Vol 2. Seattle: IASP; 1994: p.213-264.
- Simopoulos AP. Omega-3 fatty acids in health and chronic disease. Am J Clin Nutr 1999;70(suppl):560S-69S.
- Enig Mg. Know Your Fats. Bethesda Press: Silver Spring (MD): Bethesda Press; 2000: p.115.
- Cordain L. The Paleodiet. Hoboken: John Wiley & Sons; 2002.