Some doctors thrive in a personality-based clinic and have a loyal following no matter what services or equipment they offer, but for most chiropractic offices who are trying to grow and expand, new equipment purchases help us stay relevant and continue to service our client base in the best, most up-to-date manner possible. So, regarding equipment purchasing: should you lease, get a bank loan, or pay cash?
Rotavirus Update: More Adverse Reactions, Deaths Possibly Linked to Vaccinations
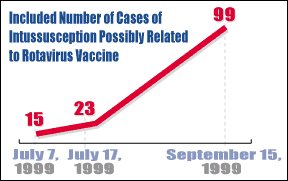
The rotavirus vaccine, which was supposed to protect small children from contracting a disease that causes severe diarrhea and vomiting, may in fact be responsible for as many as 99 cases of intussusception -including two deaths - that have been reported to the Food and Drug Administration in the past year, an FDA medical officer told reporters last month.
The number represents an enormous leap from the 15 cases that had been reported to the FDA as of July 7 and comes on the heels of a statement issued by the Centers for Disease Control urging physicians and parents to suspend all rotavirus vaccinations pending further investigation.
Intussusception, an intestinal disorder that causes one part of the bowel to fold or telescope into another, occurs most commonly in infants and small children. The disorder causes bowel obstructions leading to severe abdominal pain that can require surgery; if left untreated, the condition can be fatal.
According to Kathryn Carbone, one of the FDA's initial reviewers of the rotavirus vaccine data, all 99 cases had received at least one dose of the vaccine before developing intussusception; two children died as a result of the condition.
Originally licensed for use in the United States in August 1998, the rotavirus vaccine was expected to significantly reduce the half-million physician visits and 50,000 hospitalizations the disease causes among children in the U.S. each year. However, anecdotal reports of infants and small children developing intussusception after receiving the vaccine began to emerge almost immediately after its introduction to the general public.
By July 7 of this year, the FDA's Vaccine Adverse Events Reporting System (VAERS) had received 15 reports of intussusception out of an estimated 1.5 million doses of the rotavirus vaccine. A week later, reports from the New York Times and CNN put the number between 20 and 23 cases.
According to Carbone, the number of cases alarmed the agency because typically, only about 5-10% of all adverse events related to vaccinations are actually reported. If a minimum of 15 infant cases were reported, and if less than ten percent of the total amount of cases were actually being reported to VAERS, it would mean that the actual number of children having adverse reactions to the rotavirus vaccine would be much higher than the FDA expected.
As a result, the CDC on July 15 issued a report calling for an immediate halt to all future rotavirus vaccinations pending further investigation and advised doctors and parents to look for warning signs that could indicate intussusception.
The American Academy of Pediatrics, which had previously recommended that children receive the vaccine at two, four and six months, also called for a temporary halt to the vaccinations, stating that "initial data suggest that intussusception occurs at a younger age in vaccine recipients than in unvaccinated children."
The number of cases reported to VAERS rose dramatically after the CDC's report, Carbone said. Since each case is still under investigation, it is currently unclear whether the two child deaths - or the other 97 cases - were caused by the vaccine.
"This is very preliminary information," she said.
Meanwhile, the agency is continuing its own case-control study, paying special attention to all cases of intussusception in states whose children received the largest numbers of rotavirus vaccinations. The FDA is expected to release preliminary findings later this month.
Possible Link between Intussusception and Polio Vaccine
In a related statement, the FDA said that in addition to rotavirus, it is also investigating preliminary reports of cases of intussusception that may be related to the oral polio vaccine. Both vaccines incorporate monkey tissue in their manufacturing process, and the use of such tissue has been suggested as a causative factor in some adverse reactions to vaccination.
The oral polio vaccine, which was licensed for use in the U.S. in 1963, contains live attenuated strains of three types of polio virus which are propagated in monkey kidney cell culture. The current version of the rotavirus vaccine, meanwhile, combines human rotavirus strains with live viral strains taken from rhesus monkeys.
"We should be very careful in assuming that we can take animal strains and drop them into humans," said Dr. Bryan Roberts, an executive at Avant Immunotherapeutics in Needham, Massachusetts.
Roberts and his colleagues are currently working on a hypothesis which suggests that animal strain-based rotavirus vaccines can cause serious adverse immune reactions. Vaccines based on human viral strains can be counterbalanced by the presence of antibodies a mother passes on to her child, Dr. Roberts explained. With animal strains, however, "there is no counterbalance."
Editor's note: for more information, see "CDC Calls for Suspension of Childhood Rotavirus Vaccine" in the October 4th issue of DC (available on line at http://www.chiroweb.com/archives/17/21/04.html).



